Tyvek® for Microbial Barrier Packaging of Medical Devices & Pharmaceuticals
Article
The unique structure of Tyvek®—tough, continuous filaments—results in superior microbial barrier protection for sterilized medical instruments, devices and pharmaceuticals.
The number-one priority in selecting packaging materials for medical devices and pharmaceuticals is the ability of the package to maintain sterility from the point of sterilization until the product is opened for use. Medical and pharmaceutical packages must not only protect the contents from punctures and other damage, they must provide an effective microbial barrier. With sizes that can range from 0.002 microns to 100 microns, bacteria and viruses are a constant contamination threat to packaged medical devices and pharmaceuticals—and the health of patients.
Superior Performance
In healthcare environments where the sterility of medical devices, pharmaceuticals and supplies is critical to patient outcomes, Tyvek® has been a trusted name for more than 40 years. Even under the most rigorous, highly contaminated conditions, it can be relied on to resist penetration by bacterial spores and other contaminating microorganisms more effectively than medical-grade papers.
A One-of-a-Kind Microbial Barrier
Tyvek® is made of continuous, randomly oriented, high-density polyethylene (HDPE) filaments. This unique structure makes it harder for microbes and other contaminants to get through, giving Tyvek® greater resistance to microbial penetration than other sterile packaging materials, such as medical-grade papers.

Microbial Barrier Testing
Microbial barrier is the measure of the ability of a porous substrate to prevent bacterial spore penetration. One standard test method, ASTM F1608, measures the “filtration” efficiency of a substrate to remove spores from an aerosol being forced through the substrate in an air stream.
Tyvek® 1073B, as seen in the chart below, has an LRV of 5 and is the best porous substrate available for medical packaging.
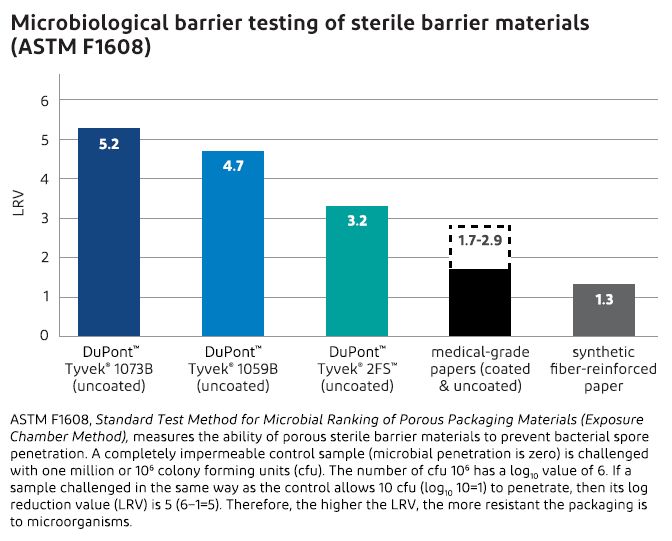
There are two disadvantages associated with ASTM F1608. The first is that the test method’s air flow rate significantly exceeds the air flow rate seen during typical distribution of a medical device. The second disadvantage is that it takes a long time to incubate the spores to get a count of how many spores penetrated the test material.
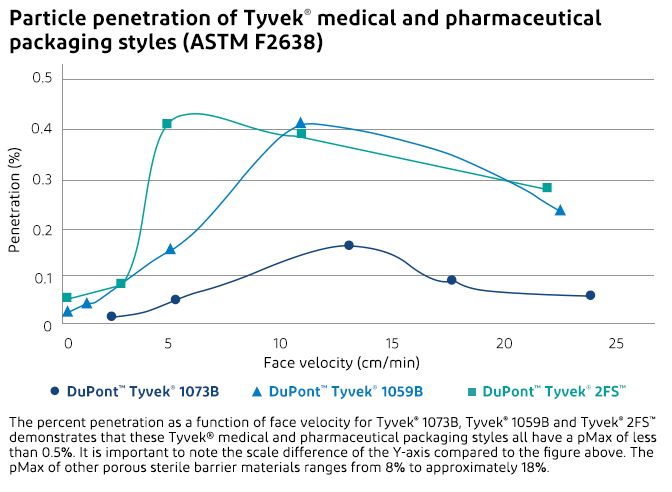
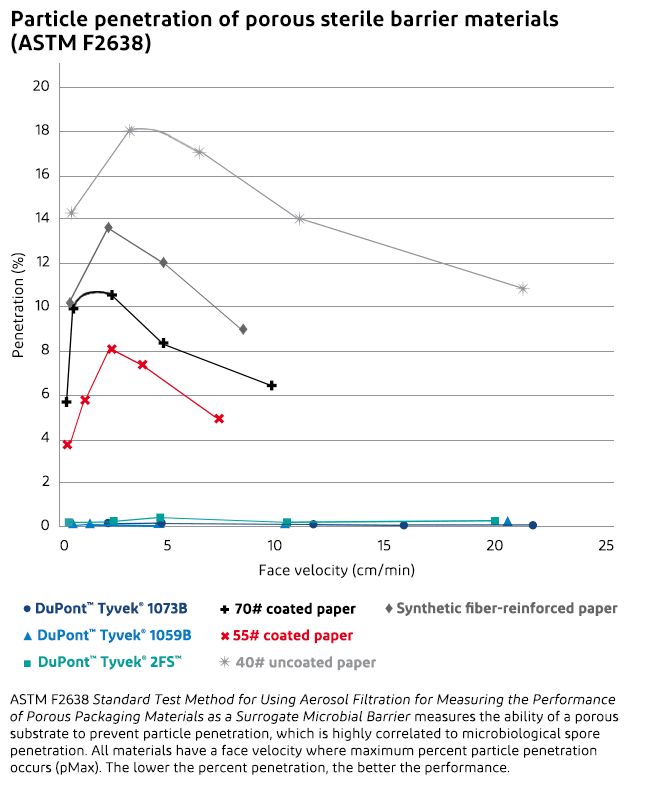
ASTM F2638 eliminates both of these problems. It is a real-time test, eliminating the need to incubate spores. It functions by counting inert particles as they penetrate the barrier material. More importantly, the air flow rates are close to those experienced during transportation, eliminating the other disadvantage. This test method also varies the flow rate and thereby generates a penetration curve. On this penetration curve, most substrates tested have a maximum. Therefore, it is possible to report a pMax, which is the maximum penetration for the given substrate. The flow rate at which the maximum occurs depends on the mass, fiber diameter and density of the substrate.
Demonstrated Longevity
A long-term shelf-life test conducted by DuPont demonstrated that packaging made with Tyvek® can maintain sterility for at least five years, providing package integrity is not compromised. The test was designed to have a more severe microbial challenge than typically found in healthcare environments. The results of the study showed that Tyvek® is a remarkably reliable microbial barrier that maintains its physical properties after aging.