Clean Peel Means Low Risk of Device Contamination
Article | February 16, 2018
When a paper lid on a sterile medical package is opened, it can release a significant number of particulates, but that’s not an issue with Tyvek®. The unique, continuous filament structure of Tyvek® results in clean peel and low lint features that minimize the risk of device contamination when packages are opened or handled.
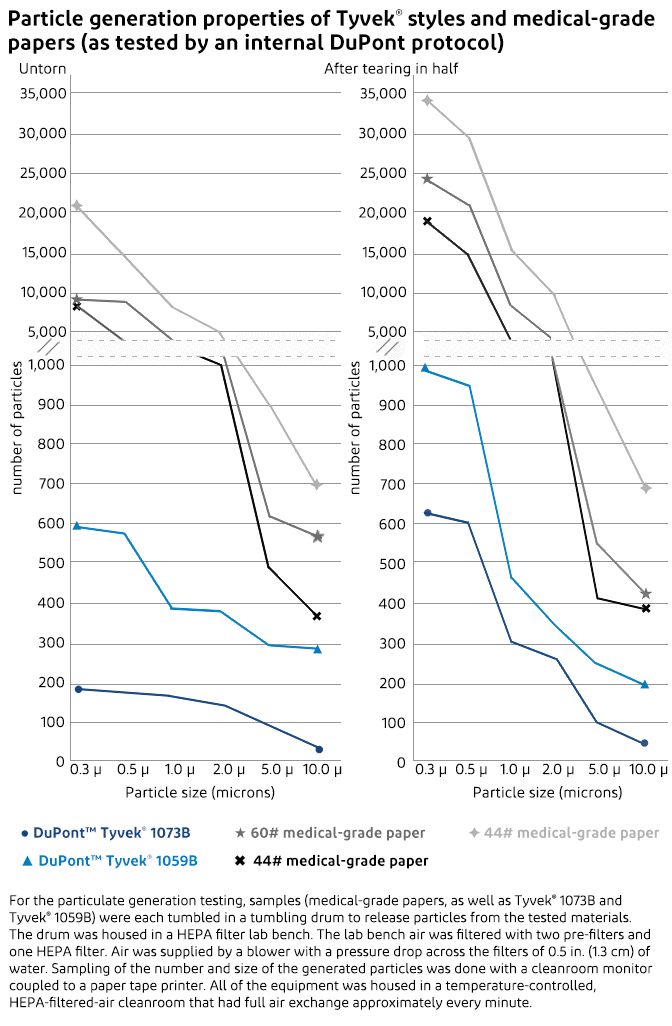
Particulate generation tests comparing Tyvek® to medical-grade papers showed conclusively that Tyvek® generates far fewer airborne particulates that could contaminate the medical device or the sterile field. The particulate generation testing measured the quantity and the size of particles generated by Tyvek® and medical-grade paper both before and after being torn in half. The tests proved that Tyvek®—with its clean peel and low-linting properties—generates significantly fewer airborne particulates than medical-grade papers.
Untorn Tyvek® generated fewer particles than medical-grade paper across the entire size range from 0.3 µ to 10.0 µ. The untorn medical-grade papers generated from 9,000 to 20,000 0.3 µ particles while Tyvek® generated less than 600. When torn, the medical-grade papers generated 19,000 to 35,000 0.3 µ particles, whereas Tyvek® generated less than 1,000.
For the testing, samples of three different medical-grade papers, Tyvek® 1073B and Tyvek® 1059B were each tumbled in a tumbling drum to release particles from the tested materials. The drum was housed in a HEPA filter lab bench. The lab bench air was filtered with two pre-filters and one HEPA filter. Air was supplied by a blower with a pressure drop across the filters of 0.5 in. (1.3 cm) of water. Sampling of the number and size of the generated particles was done with a cleanroom monitor coupled to a paper tape printer. All of the equipment was in a temperature-controlled, HEPA-filtered-air cleanroom that had full air exchange approximately every minute.